Gone are the days when abdominal surgery entailed slicing a patient open. Today, procedures can be performed with just three to four tiny incisions (usually 0.5 – 1.5cm) in the abdominal wall. Ports are inserted into these incisions through which a camera and instruments are fed.
Known as minimally invasive surgery (MIS), laparoscopic surgery or keyhole surgery, it is truly an incredible process and arguably one of the wonders of modern medicine.

A prototype of the intra-abdominal platform for surgical support (IAP) being tested during laparoscopic surgery on a model of a human abdomen
One of the instruments inserted through these ports is a laparoscopic grasper, which enables the surgeon to grasp and manipulate tissues inside the abdomen. However, David Jayne, Professor of Surgery at the University of Leeds, came up with an idea for a device that retracts the tissue without having an assistant hold the grasper during the entire procedure.
He shared this idea for a intra-abdominal platform for surgical support (IAP) with Dr Peter Culmer, associate professor in the Surgical Technologies Research Group within the School of Mechanical Engineering at the University of Leeds, who began working it into a concept.
“We got it to the point where it essentially looked a bit like an inverted umbrella and when placed through the abdominal wall would open up and effectively unfurl to provide a big scaffold that the surgeon can clip the tissue on to,” explains Culmer.
From idea to reality
He admits it was a “rough and ready prototype” that proved the device could work in principle but help would be needed to take it through to commercialisation. With a year’s worth of pilot funding from the university, Culmer began working with pd-m, a North Yorkshire-based product design consultancy.
“When we started working with the University of Leeds there was the essence of the idea but in commercial terms it needed to go through the design development cycle, which at pd-m is a very holistic approach from initial research through to a near as finished device that we could show to a commercial partner,” comments Richard Hall, managing director of pd-m.
The original intention was for the device to be made from stainless steel and for it to be reusable. However, analysing it from the perspective of manufacturing as well as functionality, Hall came to the conclusion that this would not only prove expensive to produce but the design would be compromised.
“The design was conceived as a complex structural linkage system that required multiple pins and joins in order for the geometry to collapse, go through the surgical port and then open.
“It was possible to produce this as a one-off but the reality was that as a commercial product it would have limited success. This was due to ensuring that each joint was correctly fitted which would then be used over many procedures,” explains Hall.
Back to the drawing board
Not only was the re-use of the device an issue but so was sterilisation. So, the team began to explore the possibilities of a single use device made from polymer utilising the less expensive manufacturing process of injection moulding.

The team involved in the development of the device including Dr Peter Culmer of the University of Leeds in the middle and Richard Hall of pd-m on the far right
“Although we felt that the concept we had initially come up with would work, it wouldn’t be particularly feasible commercially because of the way we had to manufacture it.
“So, we went back to the drawing board and came up with a new concept. That was quite a difficult process but really instrumental in making this something that we knew was going to work,” comments Culmer.
With the initial funding running low and recognising that there was a lot more work ahead of them, not least of all securing the intellectual property (IP), the team applied for funding from i4i (invention for innovation). Managed by the NHS National Institute of Health Research, this funding scheme supports collaborative healthcare research and development projects over a period of two years with the aim of taking a proof-of-principle concept to market.
“pd-m was actually involved in the funding proposal and I think it was seen really favourably by the people who fund the work that we were going along not just as academics but as academics in partnership with surgeons and industry partners,” says Culmer.
In fact, both Culmer and Hall stress that this close working partnership was really key to the success of the whole project. Although universities may seem an obvious place for new ideas to spring from, many of these ideas never make it to market even when academics are teamed up with industrial partners.
“Out of 100 good ideas within universities probably 97 per cent fail and that is down to people,” stresses Hall. “You can have great ideas and great IP but actually if the team members don’t respect each other and appreciate that they are all bringing something different to the party that could be a reason why good innovation could just not make it. Never underestimate the power of fostering good working relationships.”
Culmer agrees and says, “We have met very regularly throughout the project and there has been a good sense of communication, which I think has really helped the process. Although we have specifi c tasks that we are working on, we are aware of how things are progressing in other areas and it means that we can work on our tasks more eff ectively and can be quite responsive.”
Design development
Using SolidWorks, pd-m developed and engineered the CAD model. Iterative prototypes were then made to validate design detail and technical challenges. Initial partial and scale prototypes were 3D printed using pd-m’s in-house Formlabs Form 2. Later, for the more structural prototypes, the team made use of the dedicated fabrication facility for innovative robotic systems at the University of Leeds, which features a variety of prototyping tools including Objet 3D printers and laser cutters.
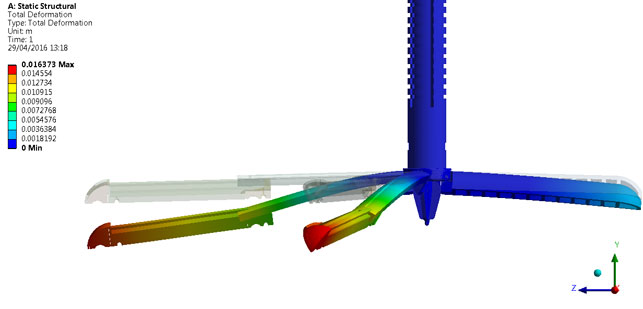
With ANSYS the team could run FEA
to look at static structural deformation
In conjunction with the CAD work and as the concepts and prototypes firmed up, Culmer and team used ANSYS for computational Finite Element Analysis (FEA) modelling to validate the device and ensure it met requirements. In particular, when they decided to switch from stainless steel to polymer, they had to make sure that the device was strong enough to support the weight of retracted tissues.
“Essentially, we run FEA to look at how it deforms under a load and make sure that stress concentrations aren’t too high,” explains Culmer. “We need to ensure it’s going to operate as it should do given the loading requirements that we’ve got.”
Throughout the development process, the project team engaged with a clinical advisory group that included surgeons and clinicians working within diff erent aspects of surgery. “We wanted to capture all of their observations and thoughts throughout the process so that the fi nal design solution was fit-for-purpose.

Calipers being used to measure the wall thicknesses
“Clearly a medical device can’t be designed by committee so it was important that the relevant and eff ective surgical aspect was embedded within the product,” comments Hall.
The clinical advisory group had a preference for stainless steel so by showing them the calculations and handing over prototypes that they could test, they managed to sway them towards polymer.
“We had to turn the opinions of the surgeons to make them understand that a single use plastic device is just as robust and functional as a metal device which will cost ten or twenty times more,” adds Hall.

Iterative prototypes were made during the design process to validate design detail and technical challenges
Learning curve
Another key aspect of the development process that the team had to undertake was production of a technical file that formally documented the entire process. This could then be handed to a commercial partner detailing all the tests that have been carried out.
“This was quite a learning curve for us within the university because it is not something we typically do,” admits Culmer.
“We could just hand across a prototype but it is much more compelling and you are more likely to get a favourable response if your documentation shows how you’ve addressed a clearly identifi able clinical need.”
The final prototype of IAP device is 25cm high and approximately 18mm in diameter, with telescopic arms that open once inside the abdominal cavity to provide a large support structure for retraction.
According to the Culmer, the project is currently at an exciting stage. Designs are frozen and the team are focused on translation of the system to a commercial partner and supporting this process through extended testing (with cadaveric models and surgical users) and technical documentation for regulatory approval.
The device was even shortlisted for the Partnership with Academia Award in the Medilink Healthcare Business Awards held earlier this year. “We felt that it was recognition of where we were at with the project and the project team too,” says Culmer.
“We’ve achieved quite a lot with this working arrangement and now we have the commercial interest too. It’s been really good to see that partnership go all the way through.”
pd-m.com
leeds.ac.uk

How a pioneering medical device became a commercial reality
Default






