Portable protection
Using its new EPIC sensor, electronics company Plessey has developed an easy to use, portable device to detect changes in electric potential fields in the heart rate.

The Plessey imPulse is a device that allows users to place their two thumbs on the sensor pads and have an accurate trace taken and instantly displayed wirelessly on a nearby screen, such as a smart phone.
The product helps users complete a routine, quick and accurate recording of ECG (electrocardiogram) signals outside of the medical environment and without the need for conductive gel or skin preparation.
The realisation of the project was undertaken by Cheltenham-based designers Square Banana.
“We analysed the changing consumer, technology and medical markets to establish a clear direction for the product concept,” says Square Banana’s founder Russell Beard.
“We started with sketchwork, but moved very quickly into SolidWorks to develop a tight ergonomic envelope suited to the brief and which formed the basis of a prototype assembly, containing existing components.”
In the first weeks, following the first 3D CAD assembly, the model was sent to Square Banana’s prototyping suppliers from which a batch of 15 complete working products were created.
CNC, SLA, PU Vacuum casting and a variety of finishing techniques were used to create prototypes that could effectively simulate a final product for sales teams to promote and technical teams to test simultaneously.
With the imPulse well on its way to production, the team were then engaged to design a number of other concepts for the EPIC sensor.
The Plessey inCite is a wearable monitor, worn like a wristwatch, allowing users to view their ECG at any given time.
This example would be an entry-level product, with straightforward usability and a peripheral lighting ring to indicate progress and feedback.
The near-future concept was designed in much the same manner, utilising Keyshot for the rendering work to present accurate imagery to Plessey for future development.
Further products would start to integrate more complex display technologies and a greater number of health related features as technology ultimately shrinks to allow for such wearable tech.
Anchoring the issue
Intravenous therapy is commonplace during hospital stays, yet the way tubes are secured means they can still tug and move, causing increased risk of infection.
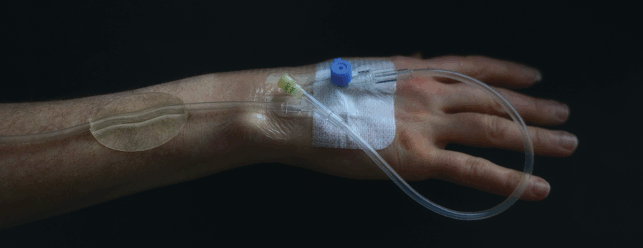
Professor of endocrinology and honorary consultant physician at The University of Bristol, Professor Andrew Levy, recognised that a simple, cheap and disposable component could overcome this and started working on sketches and handmade prototypes in his home workshop.
Quickly he realised that fine control over the material and geometry of the device was critical to developing his idea.
This led him, via innovation support company CareTechnica, to Amalgam Modelmaking, in order to finetune his Tube Anchor idea – a simple one-piece device that addresses many of the problems in a single step solution.
Its transparent form allows a clear view of the skin underneath; a clean, smooth, trap-free shape for hygiene, and tubes can easily be removed and replaced.
Amalgam produced several options in SolidWorks to explore some of the issues. Having fine-tuned the design after several 3D printed iterations, the finished 3D prints were used as master models for soft tooling and resin vacuum casting in various elastomers.
“It is hard to see how this product could have ever been refined to the degree required without the ability to quickly and cheaply test physical models along the way,” says Mike Harvey, director of Amalgam Modelmaking.
A small batch of functional prototypes were used on volunteers for feedback from nurses and patients, which in-turn led to five cycles of tweaks to get everything perfect.
At this point CareTechnica took over the legal and promotional side of the process, licensing it to P3 Medical, which will launch the product shortly.
Getting to the bottom of the problem
With the aim of improving products that are not user friendly, un-hygienic or even potentially dangerous, Aquaflush Medical Limited was set up as a joint-venture between product design agency Product Resolutions, a medical device manufacturer and a consultant.
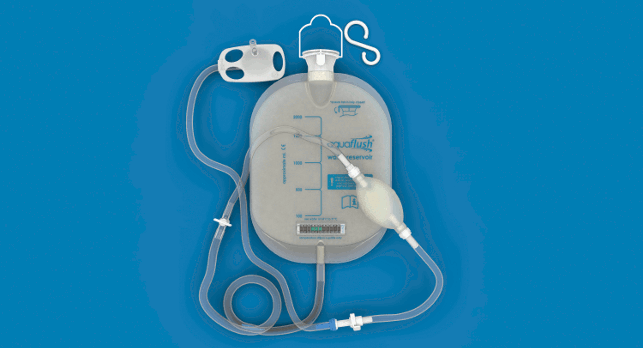
Its design for the Aquaflush anal irrigation range includes custom-designed valves and connectors and a single-use cone catheter made from paper pulp.
“The first stage was talking to the people that actually use these products and understanding what was wrong with what was available,” says Paul Robbins, director at Product Resolutions.
“We then used a combination of sketching and 2D illustrations to create schematic diagrams of the process and the components required for the finished device.”
SolidWorks was used to build the 3D model, yet the nature of the device meant that the only way to test the product was to make samples, test them on patients, refine and repeat the process.
Product Resolutions initially used its in-house Makerbot 2X to help with the development before sending the CAD data to its prototype supplier in China, which CNC machined critical parts like valves and connectors.
“The most challenging part technically was probably the cone, valve and catheter assembly,” says Robbins. “This is the part that actually goes into the body and we had to work very closely with the pulp-paper manufacturer to ensure they could get the quality and tolerance we needed.
“We literally had to turn their tooling process inside-out to make it work for us!”
We take a look at three new healthcare products
Default






