
RapidRhythm is a breakthrough technology that can screen heart health in under 90 seconds
Heart patients could be screened in just 90 seconds using a wireless, handheld device now in development. More than half a million people in the UK suffer from atrial fibrillation (AF), which causes an irregular heart rate, and identifies a high risk of stroke. AF results in 575,000 hospital admissions a year and more than 900,000 people live with the after effects of stroke.
The current method of screening is an electrocardiogram (ECG) test, for which patients undress and lie down to have ten electrodes connected to their body by a qualified medical professional. The procedure can take up to 20 minutes.
The RapidRhythm device is held to a patient’s chest to diagnose AF and other major cardiac conditions. Data is relayed to a LCD touchscreen displaying a high resolution, multichannel ECG trace and wirelessly downloaded for further analysis by a physician.
Design collaboration
The latest version of RapidRhythm has been developed by product design consultancy AME Group and electronics and software developer Practical Control.
The device conducts a full 8 or 12-lead ECG measurement and can be used in a number of clinical settings. It gives the user high-definition video guidance on how to conduct the test, requires less training time for GPs, doctors and nurses than traditional ECG machines and can be used without the need for patients to undress.
The device can also securely transfer the ECG recording to a remote interpretation service or nearby printer for on-site analysis.
AME’s designers have developed an ergonomic solution that enables a range of electrode types to be easily attached to the device and applied to the patient. Once the test has been completed the electrodes are removed at the touch of a button.
Patients have been involved throughout the user-focused design process. AME conducted on-site interviews at GP surgeries and heart wards to observe users and understand current ECG tests in order to find problems and opportunities for innovation.
The findings identified a number of unique product features that were explored across a range of design concepts. These include a single-handed electrode application process, an automatic electrode release system, ergonomic form and obvious grip for left or right handed use.

AME Group worked with medical professionals and carried out extensive in-field studies
Developing the design
These concepts were presented to patients and healthcare professionals to identify a preferred design. The chosen product was engineered in 3D CAD, with a number of test prototypes created using AME’s inhouse facilities.
AME’s low volume production facilities will be used to manufacture a small quantity of the devices, which will then be used to validate the product in the field.
Tim Stern, design manager at AME Group, said: “Observing and understanding the environment in which products are used can have a significant impact on the design output so we took time to fully understand users and patients and how they engaged with existing ECG products.
“The findings were integral to how we explored and developed the aesthetics, features and ergonomics of the RapidRhythm device. Prototyping is essential to the development process and enables our engineers to assess a design, highlight issues and minimise the risk and potential costs of resolving them at a later date.”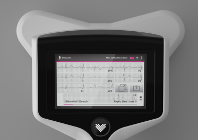
AME Group helps bring an ECG device to market
Default






